Encor Rabbit Polyclonal to GAPDH
Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) is a metabolic enzyme responsible for catalyzing one step in the glycolytic pathway, the reversible oxidative phosphorylation of glyceraldehyde 3-phosphate. Because GAPDH as a protein is expressed in large amounts and which is required at all times for an important “house keeping” functions, levels of GAPDH mRNA are often measured and used as standards in studies of mRNA expression. Increasingly, scientists are making use of specific antibodies to GAPDH in comparable studies of levels of protein expression. This antibody can be used as a loading control for western blotting experiments, allowing comparison between the level of this protein and others in a cell or tissue. Apart from a role in glycolysis, GAPDH may have other roles such as in the activation of transcription (1). GAPDH is reported to bind to a variety of other proteins, including the amyloid precursor protein, mutations in which cause some forms of Alzheimer’s disease, and the polyglutamine tracts of Huntingtin, the protein product aberrant forms of which are causative of Huntington’s disease (2,3). Associations with actin and tubulin have also been reported. The protein may also have a role in the regulation of apoptosis, and interestingly migrates from the cytoplasm into the nucleus when cells become apoptotic (4). The immunogen used to raise this particular antibody was extensively purified pig GAPDH. As with our monoclonal antibody to GAPDH, MCA-1D4, this antibody can be used as a western blotting standard. See references 5-25 for the use of MCA-1D4 in this way. The HGNC name for this protein is GAPDH.
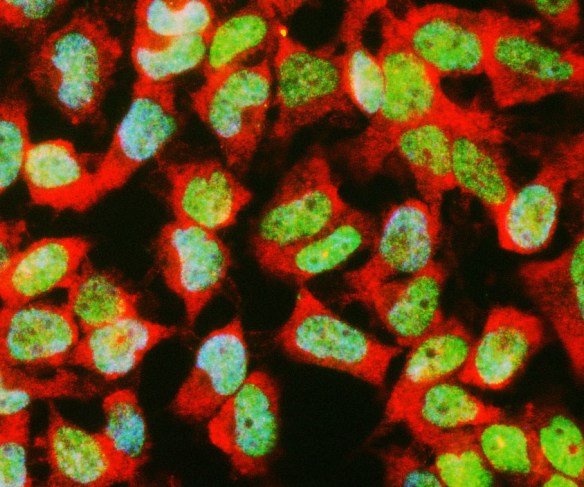
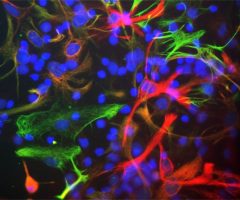
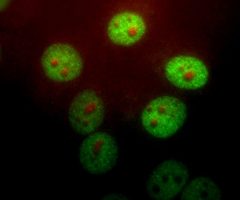
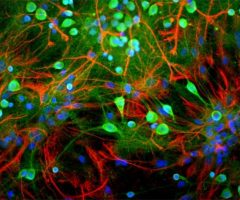
Antibody was raised in rabbit against extensively purified pig GAPDH. The antibody is provided in the form of crude rabbit serum. This antibody is known to react with GAPDH from human, cow, mouse, rat and other mammals. RPCA-GAPDH specifically recognizes GAPDH both in western blots and in immunocytochemical experiments. On blots RPCA-GAPDH reveals a prominent ~36kDa band, and on cells in tissue culture the antibody stains in a punctate cytoplasmic fashion. Since GAPDH is one of the most conserved proteins known, it is likely that the antibody is effective on other species also.
HGNC name(s) : GAPDH
Host : Rabbit
Clonality : Polyclonal
ID : EnCor Biotechnology Glyceraldehyde 3-Phosphate Dehydrogenase GAPDH
Reactivity : Human | Horse | Cow | Pig | Chicken | Rat | Mouse
Isotype : IgG
Conjugation : none
Immunogen : Porcine GAPDH
Mass of detected protein : 38 kDa
Uniprot ID : P04406
KGNC name : GAPDH
RRID # : AB_2572289
Purification : Serum
Storage : Shipped on ice. Store at 4°C. For long term storage, leave frozen at -20°C. Avoid freeze / thaw cycles.
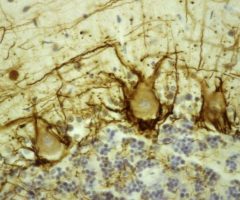
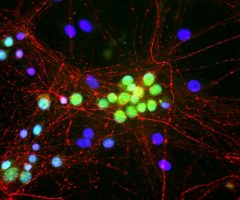
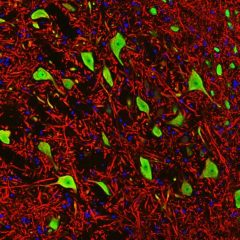
Validated applications : WB | IF/ICC | IHC
Suggested Dilutions:
WB: 1:30 000
IF/ICC and IHC: 1:10 000
References :
1. Morgenegg G, Winkler GC, Hubscher U, Heizmann CW, Mous J, Kuenzle CC. Glyceraldehyde-3-phosphate dehydrogenase is a nonhistone protein and a possible activator of transcription in neurons. J Neurochem. 47:54-62 1986
2. Schulze H, Schuler A, Stuber D, Dobeli H, Langern H & Huber G. Rat brain glyceraldehyde-3-phosphate dehydrogenase interacts with the recombinant cytoplasmic domain of Alzheimer’s beta-amyloid precursor protein. J Neurochem. 60:1915-22 1993
3. Burke JR, Enghild JJ, Martin ME, Jou Y-S, Myers RM, Roses AD, Vance JM & Strittmatter WJ. Huntingtin and DRPLA proteins selectively interact with the enzyme GAPDH. Nature Med. 2: 347-350, 1996.
4. Dastoor Z. & Dreyer, J-L. Potential role of nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase in apoptosis and oxidative stress. J. Cell Sci. 114:1643-1653 2001.
5. Fortun J, Dunn WA, Joy S, Li J. & Notterpek, L. Emerging Role for Autophagy in the Removal of Aggresomes in Schwann Cells. J. Neurosci. 23:10672-10680 2003.
6. Ellis RC, Earnhardt JN, Hayes RL, Wang KK & Anderson DK. Cathepsin B mRNA and protein expression following contusion spinal cord injury in rats. J Neurochem. 88:689-97 2004.
7. Fortun J, Li J, Go J, Fenstermaker A, Fletcher BS & Notterpek L. Impaired proteasome activity and accumulation of ubiquitinated substrates in a hereditary neuropathy model. J Neurochem. 92:1531-41 2005
8. Fortun J, Li J, Go J, Fenstermaker A, Fletcher BS & Notterpek L. Impaired proteasome activity and accumulation of ubiquitinated substrates in a hereditary neuropathy model. J Neurochem. 93:766-8 2005
9. Iskandar M, Swist E, Trick KD, Wang B, L’Abbe MR, Bertinato J. Copper chaperone for Cu/Zn superoxide dismutase is a sensitive biomarker of mild copper deficiency induced by moderately high intakes of zinc. Nutr J. 4:35 2005
10. Fortun J, Go JC, Li J, Amici SA, Dunn WA Jr, Notterpek L. Alterations in degradative pathways and protein aggregation in a neuropathy model based on PMP22 overexpression. Neurobiol Dis. 22:153-164 2006
11. Amici SA, Dunn WA, Murphy AJ, Adams NC, Gale NW, Valenzuela DM, Yancopoulos GD & Notterpek L.Peripheral Myelin Protein 22 Is in Complex with {alpha}6beta4 Integrin, and Its Absence Alters the Schwann Cell Basal Lamina. J. Neurosci. 26:1179-89 2006
12. Amici SA, Dunn WA & Notterpek, L. Developmental abnormalities in the nerves of peripheral myelin protein 22-deficient mice. J. Neurosci. Res. 85:238-249 2006
13. Felitsyn N, Stacpoole, PW & Nottepek L. Dichloroacetate causes reversible demyelination in vitro: potential mechanism for its neuropathic effect. J. Neurochem 100:429-36 2007
14. Rangaraju S, Madorsky I, Pileggi JG, Kamal A, Notterpek L. Pharmacological induction of the heat shock response improves myelination in a neuropathic model. Neurobiol Dis. 32:105-15 2008
15. Felitsyn N, McLeod C, Shroads AL, Stacpoole PW, Notterpek L. The heme precursor delta-aminolevulinate blocks peripheral myelin formation. J Neurochem. 106:2068-79 2008
16. Lau P, Verrier JD, Nielsen JA, Johnson KR, Notterpek L, Hudson LD. Identification of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J Neurosci. 28:11720-30 2008
17. Verrier JD, Lau P, Hudson L, Murashov AK, Renne R, Notterpek L. Peripheral myelin protein 22 is regulated post-transcriptionally by miRNA-29a. Glia 57:1265-79 2009
18. Rangaraju S, Hankins D, Madorsky I, Madorsky E, Lee WH, Carter CS, Leeuwenburgh C, Notterpek L.Molecular architecture of myelinated peripheral nerves is supported by calorie restriction with aging. Aging Cell. 8:178-91 2009
19. Madorsky I, Opalach K, Waber A, Verrier JD, Solmo C, Foster T, Dunn WA Jr, Notterpek L. Intermittent fasting alleviates the neuropathic phenotype in a mouse model of Charcot-Marie-Tooth disease. Neurobiol Dis. 34:146-54 2009
20. Opalach K, Rangaraju S, Madorsky I, Leeuwenburgh C, Notterpek L. Lifelong calorie restriction alleviates age-related oxidative damage in peripheral nerves. Rejuvenation Res. 13:65-74 2010.
21. Verrier JD, Semple-Rowland S, Madorsky I, Papin JE, Notterpek L. Reduction of Dicer impairs Schwann cell differentiation and myelination. J. Neurosci Res. 88:2558-68 2010.
22. Zeier Z, Madorsky I, Xu Y, Ogle WO, Notterpek L, Foster TC. Gene Expression in the Hippocampus: Regionally Specific Effects of Aging and Caloric Restriction. Mech Ageing Dev. 132:8-19 2011
23. Verrier JD, Madorsky I, Coggin WE, Geesey M, Hochman M, Walling E, Daroszewski D, Eccles KS, Ludlow R, Semple-Rowland SL. Bicistronic lentiviruses containing a viral 2A cleavage sequence reliably co-express two proteins and restore vision to an animal model of LCA1. PLoS One. 6:e20553 2011
24. Lee WH, Kumar A, Rani A, Herrera J, Xu J, Someya S, Foster TC. Influence of viral vector-mediated delivery of superoxide dismutase and catalase to the hippocampus on spatial learning and memory during aging. Antioxid Redox Signal. 16:339-50 2012
25. Kumar A, Rani A, Tchigranova O, Lee WH, Foster TC. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiol Aging. 2011 Aug 3. [Epub ahead of print]
CiteAb citations- this will find papers in which the EnCor antibody was purchased from us directly, but will not find citations to our antibodies when purchased through our numerous OEM partners.
Additional information
| Format | 50 ul, 100 ul, 500 ul |
|---|---|
| Supplier | |
| Host | Rabbit |
| Clonality | Polyclonal |
| Reactivity | Chicken, Cow, Horse, Human, Mouse, Pig, Rat |
| Validated Applications | WB, IHC, IF/ICC |
| Conjugation | None |
| Isotype | IgG |
Ask a question about Glyceraldehyde 3-Phosphate Dehydrogenase Antibody – RPCA-GAPDH
You must be logged in to post a review.


Reviews