Encor Rabbit polyclonal to Anti-α-II-spectrin / α-Fodrin / SPTAN1
The spectrin family of proteins were originally discovered as major components of the submembranous cytoskeleton of osmotically lysed red blood cells (1). The lysed blood cells could be seen as clear red blood cell shaped objects in the light microscope and were referred to as red cell “ghosts”. The major proteins of these ghosts proved to be actin, ankyrin, band 4.1 and several other proteins, including two major bands running at about 240 kDa and 260 kDa on SDS-PAGE gels. This pair of bands was named “spectrin” since they were discovered in these red blood cell ghosts (1). Later work showed that similar high molecular bands were seen in membrane preparations from other eukaryotic cell types.
Work by Levine and Willard described a pair of about ~240-260 kDa molecular weight bands which were transported at the slowest rate along mammalian axons (2). They named these proteins “fodrin” as antibody studies showed that they were localized in the sheath under the axonal membrane, but not in the core of the axon (2, fodros is Greek for sheath). Subsequently, fodrin was found to be a member of the spectrin family of proteins, and the spectrin nomenclature is now normally used (3).
Spectrins form tetramers of two α and two β subunits, with the α corresponding to the lower molecular weight ~240 kDa band and the β corresponding to the ~260 kDa or in some case much larger band. Most spectrin tetramers are about 0.2 microns or 200 nm long, and each α and β subunit has a cell type specific expression pattern. The basic structure of each spectrin subunit is the spectrin repeat, which is a sequence of about 110 amino acids which defines a compact domain contain three closely packed α-helices. Each spectrin subunit contains multiple copies of this repeat, with 20 in each of the α subunits. The β I-IV subunits each contain 17 spectrin repeats, while the β V subunit, also known as β-heavy spectrin, contains 30 of these repeats.
The various subunits also contain several other kinds of functional domains, allowing the spectrin tetramer to interact with a variety of protein, ionic and lipid targets. The α-subunits each contain one calmodulin like calcium binding region and one Src-homology 3 (SH3) domain, an abundant domain involved in specific protein-protein interactions. The β subunits all have a N-terminal actin binding domain and may also have one SH3 domain and one pleckstrin homology domain, a multifunctional type of binding domain which in β I spectrin at least binds the membrane lipid PIP2 (5).
Spectrins are believed to have a function in giving mechanical strength to the plasma membrane since the tetramers associate with each other to form a dense submembranous geodesic meshwork (3). They also bind a variety of other membrane proteins and membrane lipids, and the proteins they bind to are therefore themselves localized in the membrane. Diseases may be associated with defects in one or other of the spectrin subunits (6). For example, some forms of hereditary spherocytosis, the presence of spherical red blood cells which are prone to lysis, can be traced to mutations in some of the spectrin subunits (7). As another example, various mutations altering either the actin binding domain or repeat three of bIII spectrin are causative of one form spinocerebellar ataxia, SCA5 (8).
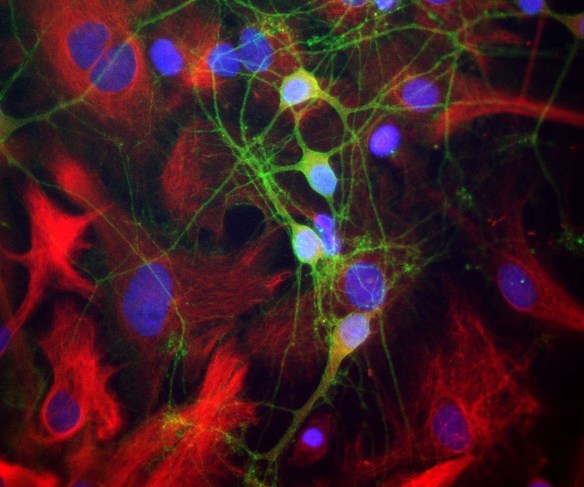
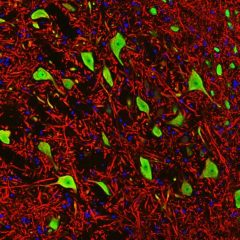
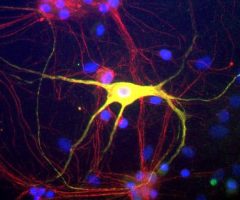
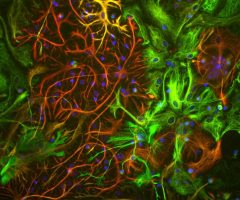
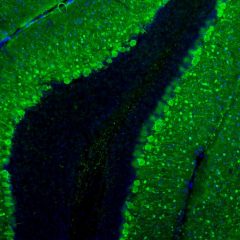
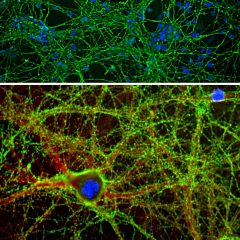
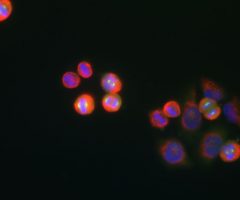
The α-II subunit is widely expressed in tissues but, in the nervous system, is found predominantly in neurons. Our antibody can therefore be used to identify neurons and fragments derived from neuronal membranes in cells in tissue culture and in sectioned material. The antibody was raised against a mix of five recombinant constructs containing the entire C-terminal region of human alpha-II spectrin (amino acids 676-2,400). The 9th spectrin repeat also includes a Src-homology 3 domain, and the extreme C-terminus beyond the 20th spectrin repeat contains two EF hand calcium binding motifs. These constructs were expressed in and purified from E. coli. The HGNC name for this protein is SPTAN1.
HGNC name(s) : SPTAN1
Host : Rabbit
Clonality : Polyclonal
ID : EnCor Biotechnology α-II spectrin all-Spec
Reactivity : Human | Horse | Cow | Pig | Chicken | Rat | Mouse
Isotype : IgG
Conjugation : none
Immunogen : Recombinant human construct aa 676-2,400
Mass of detected protein : 240 kDa
Uniprot ID : Q13813
KGNC name : SPTAN1
RRID # : AB_2572382
Purification : Serum
Storage : Shipped on ice. Store at 4°C. For long term storage, leave frozen at -20°C. Avoid freeze / thaw cycles.
Validated applications : WB | IF/ICC | IHC
Suggested Dilutions:
WB: 1:5 000-1:10 000. ICC/IF and IHC: 1:500-1:1 000 .
References :
1. Marchesi VT & Steers E Jr. Selective solubilization of a protein component of the red cell membrane. Science 159:203-4 (1968).
2. Levine J & Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 90:631-42 (1981).
3. Bennett V & Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 81:1353-92 (2001).
4. Djinovic-Carugo K, Gautel M, Ylänne J & Young P. The spectrin repeat: a structural platform for cytoskeletal protein assemblies. FEBS Lett. 513:119-23 (2002).
5. Wang, DS and Shaw G. The association of the C-terminal region of beta I sigma II spectrin to brain membranes is mediated by a PH domain, does not require membrane proteins, and coincides with a inositol-1,4,5 triphosphate binding site. BBRC 217:608-15 (1995).
6. Bennett V & Healy J. Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol Med 14:28-36 (2008).
7. Eber S & Lux SE. Hereditary spherocytosis–defects in proteins that connect the membrane skeleton to the lipid bilayer. Semin Hematol 41:118-41 (2004).
8. Ikeda Y. et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nature Genetics 38:184-90 (2006).
Additional information
| Format | 50 ul, 100 ul, 500 ul |
|---|---|
| Supplier | |
| Host | Rabbit |
| Clonality | Polyclonal |
| Conjugation | None |


Reviews